Projects
Embryo-maternal interactions at the implantation site

The implantation of the early embryo into the uterine wall is a key step of the reproductive cycle in mammals. This critical process mediates the connection of the conceptus to the maternal tissues during the early stages of pregnancy. Despite the advances in the assisted reproductive technologies, the majority of the IVF procedures are not effective mainly as a result of implantation failure. Implantation defects that compromise embryonic development account for almost half of the total pregnancy failures in the human assisted reproduction. However, the regulatory mechanism of implantation and the factors governing discontinuation of pregnancy at this early phase of development are poorly understood. Using biomimetic platforms, we aim to model and decipher the crosstalk between the invading mouse embryo and the maternal environment in order to understand the process of implantation.
Embryo dormancy and activation
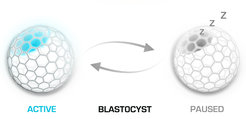
Failure of implantation does not necessary ends up bad for the embryo. In the absence of ovarian estrogen the blastocyst cannot attach to the uterus, as the uterine surface is rendered non-adhesive. What happens next with the embryo is a fascinating phenomenon that is not well understood. Instead of dying and vanishing, the blastocyst enters a state of dormancy (diapause) that can last for couple of weeks in mice and several months in other mammals. Anytime a peak of estrogen can quickly render the uterine endometrium in a receptive state that allows a delayed implantation to take place. Induction of embryo dormancy is used as adaptive response to environmental conditions, as well as reproductive strategy by many mammalian species. Our goal is to dissect the embryo intrinsic mechanisms that underline the initiation and maintenance of diapause, as well as the subsequent blastocyst activation and re-entry into embryogenesis.
Blastocyst to egg cylinder transition

The process of implantation is a gateway for subsequent embryonic development. This process is characterized with major reorganization of the tissues of the developing conceptus. Between E4.5 and E5.5, just within 24 hours, the mouse embryo is reshaped from a blastocyst into an emerging egg cylinder. These fundamental changes in the morphology of the embryo coincide with burst of cell proliferation and reorganization of the transcriptional, epigenetic, metabolic and signalling states of both the pluripotent and the supportive extraembryonic lineages. As the maternal tissues engulf and conceal the embryo, these events are largely unexplored. Here we want to understand how the developmental program changes gears to shift from pre- to post-implantation embryogenesis, which factors set “points of no return” driving this transition forward and what are the mechanisms that synchronise all cellular processes into a balanced ontogenetic flow.
Self-organization of the pluripotent lineage
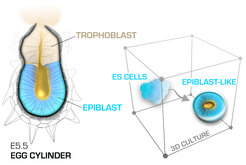
At blastocyst stage the pluripotent epiblast is an amorphous ball of cells that gets reorganized during the peri-implantation stages into a polarized cup-shaped epithelium with central (proamniotic) cavity. At the same time, the transiently established naïve pluripotency of the pre-implantation epiblast is dismantled, transforming into a more developmentally advanced post-implantation pluripotent state. This transition can be faithfully recapitulated using 3D culture of mouse ES cells, representing an in vitro model of epiblast development. Using this model system, in combination with direct embryo micromanipulations, we seek to understand how the pluripotent states control the cell shape, what are the key signalling pathways that execute the epithelial program and what role the overall architecture of the epiblast plays in the communication and patterning of the early cell lineages.
Biomimetic platforms
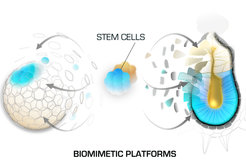
Working with mouse embryos enables direct analysis of developmental processes, however this in vivo approach has its limitations such as the low number of cells that the embryos can provide for downstream analysis and the long time required for the generation of transgenic mouse strains. To overcome these limitations, we are constantly working on the establishment and refinement of novel biomimetic platforms that incorporate embryos, stem cells and 3D culture environments to recapitulate developmental processes in vitro. We use these model systems in combination with large set of methods such as live imaging, genomic engineering, next generation sequencing techniques, proteomics, functional screens etc. This approach provides the necessary material and experimental toolbox for in depth mechanistic studies that complement our observations in vivo.




